What the BCMA CAR-T Adcom Revealed About the Field's Future
Below I sink my teeth into the commentary, arguments, and results from Friday (March 15th’s) FDA ODAC meeting regarding sBLA of Ide-Cel and Cilta-Cel in 2L-4L R/R-MM
Hello reader! Your sponsorship could go right here at the very top of the next Big Pharma Sharma post. A sponsored post is 100% accessible to all subscriber levels and gets your brand, product, or service in front of my audience of BioPharma Industry decision-makers. If you or your company is interested in becoming a Big Pharma Sharma sponsor, please reach out to me here or on my socials. You can learn more about sponsored posts on the Sponsored Posts page (link).
If you were tapped into biotech twitter and heme/onc twitter towards the end of last week, you would have been inundated with play-by-plays from KOLs, analysts, and traders about the FDA’s join ODAC meeting, to solicit recommendations from a panel of experts on the sBLA’s for the two leading BCMA CAR-T therapies in earlier lines of therapy.
Spoiler alert, the FDA advisory committee voted in favor of both BCMA CAR-Ts (11-0 for J&J/Legend’s CARVYKTI/Cilta-Cel in 2L-4L; 8-3 for BMS/TwoSeventy’s ABECMA/Ide-cel in 3L-4L) being approved in their proposed indications. This represents a meaningful step as it significantly increases the likelihood of approval for both drugs and expansion of their total addressable market from the smaller 5th-line setting to the much larger 2L-4L pool. While the votes don't guarantee approval, the FDA rarely goes against their Adcom panel opinions.
Rather than recount the play-by-play, I think the implications from key discussion topics are more fascinating to unpack, speculating on how they readthrough to the BCMA CAR-T landscape and prospects of these two therapies.
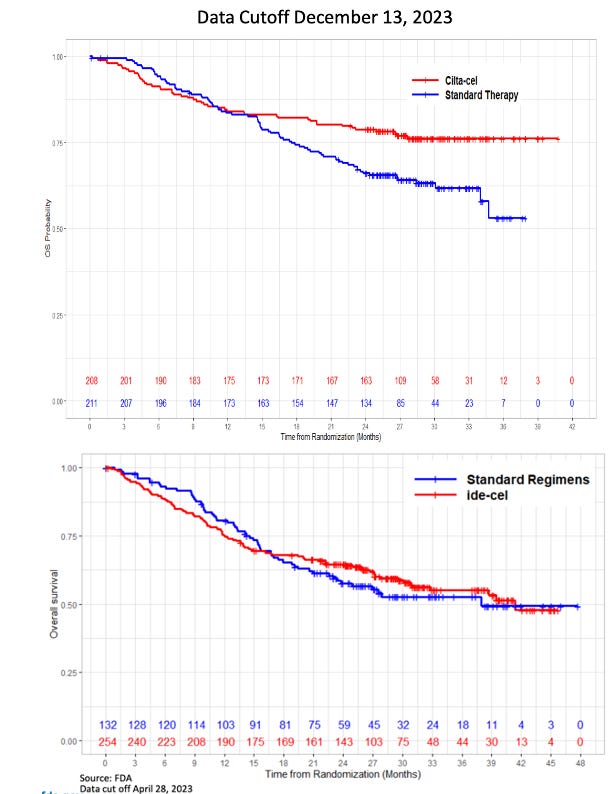
Note: You can access all the ODAC materials here if you’d like to take a deeper dive. I’m not going to recap the data from the two pivotal studies in question in detail, since we’ve largely seen these data sets already.
The quiet part out loud: early deaths are because both products take way too long to manufacture and deliver
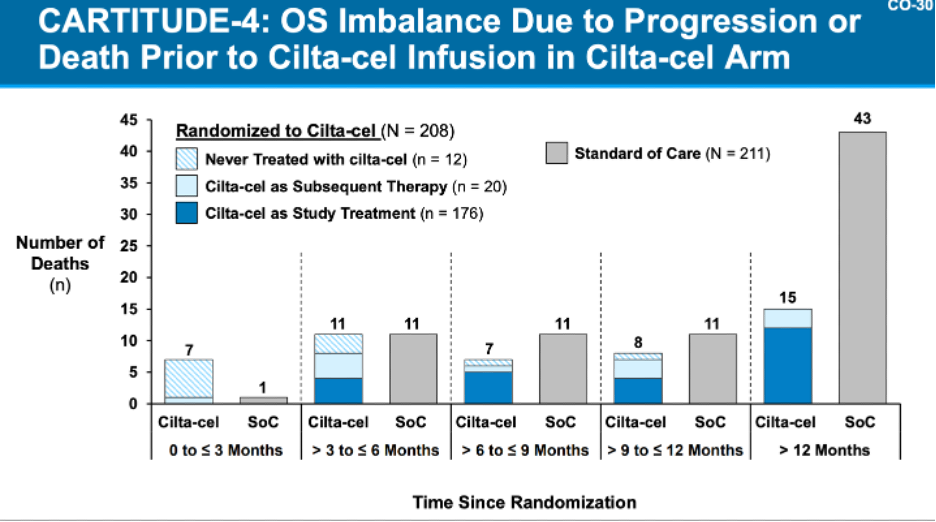
In both FDA analyses, the reviewers called out the increased rates of early deaths on study in the CAR-T arms. This caused the survival curves of both products to start off lower than the SoC arms and then eventually cross-over over time. Ide-Cel data showed 18% of patients died within the first 9 months vs. 11% for the comparator arm, but 8% of those dates came from patients who never received Ide-cel. Similarly, for cilta-cel, J&J’s data showed that the survival imbalance was largely due to patients progressing or dying before receiving cilta-cel. Notably for cilta-cel, 4% of deaths in the cilta-cel arm were due to COVID-19, as this study took place during the pandemic, and will obviously not be as much of factor in the current environment.
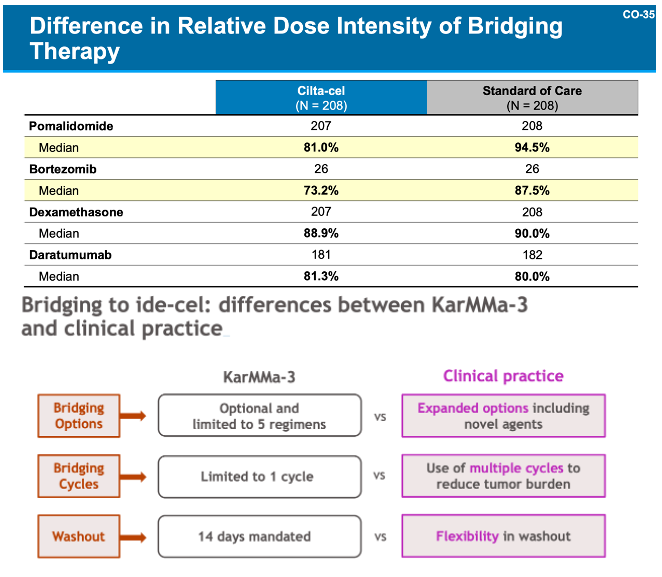
These deaths were explained by both companies similarly, as both sponsors highlighted that the predominance of deaths occurred in patients who never received CAR-T, to wit, the patients that died early on were those who progressed while waiting for their CAR-T treatment (more on this in a little bit). Both companies highlighted inadequate bridging therapy in the context of the clinical trials as the primary reasons for progression, noting that in the real-world setting, physicians would have more flexibility in terms of bridging options and dose to keep disease controlled while CAR-Ts are being manufactured.