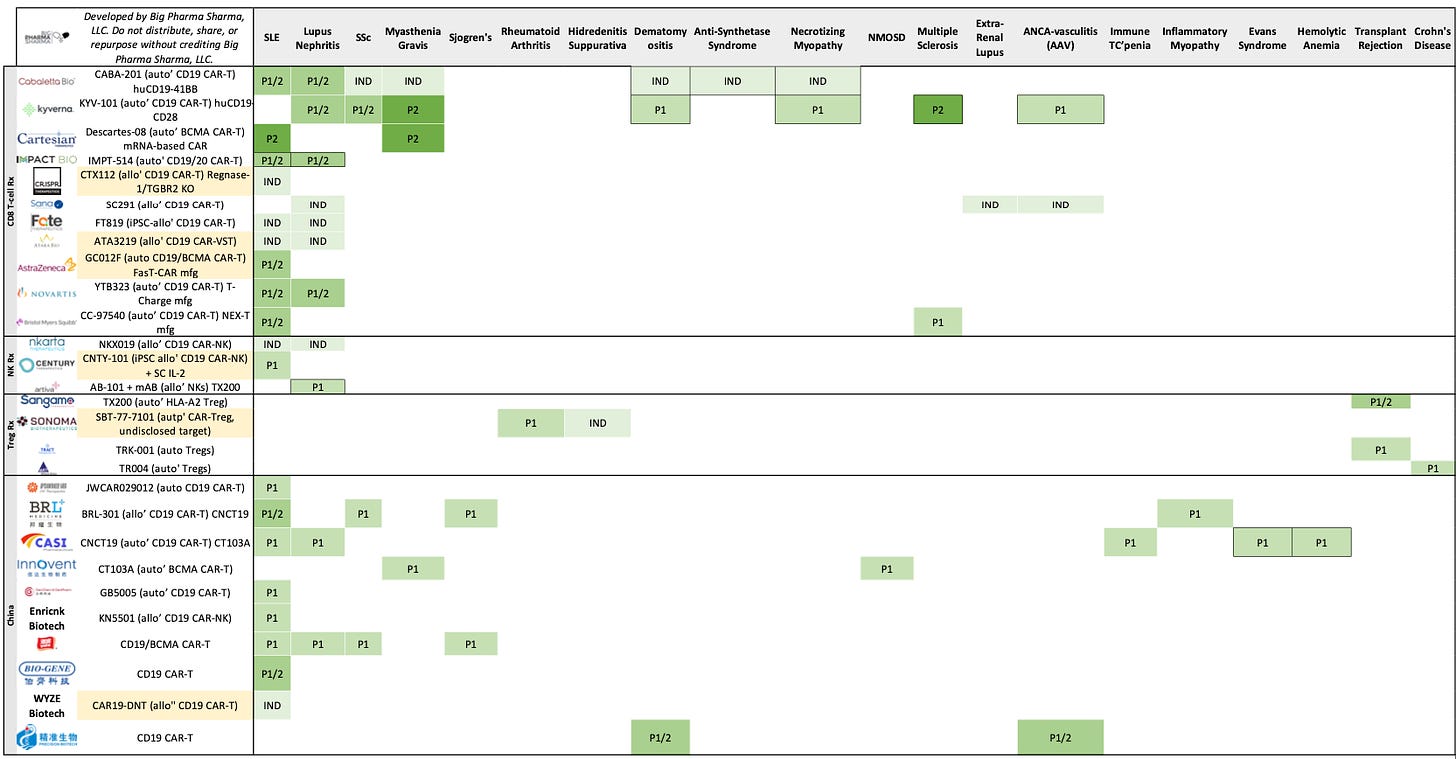
Updated Cell Therapy Autoimmune Landscape
I updated the autotimmune cell therapy landscape I put together back in November 2023 and teased out some new insights given the developments in the field.
Introduction
With Kyverna’s recent mega IPO and many other cell therapy companies announcing updates in their autoimmune ambitions, I thought it was time to update my previously published development map on this topic. To be honest, I wasn’t expecting to update this map until Q2 2024, but in researching the landscape, there has already been significant movement in the ~4-months or so since my last post that warrants a refresh. Because of all of you getting the word out about Big Pharma Sharma, our community has seen significant growth in paid subscribers since the last version of this landscape. First off, thank you for your support and advocacy. Secon of all, this landscape was one of my most popular posts and now is as perfect a time as ever to update this landscape for the many new joiners to the BPS subscriber community. To catch up on my original post, I’ve hyperlinked it here.
Development Map Orientation
Below you’ll see a graphic or ‘development map’ of sorts that captures cell therapy programs in development for autoimmune diseases. The map captures what phase of development each program is for the respective autoimmune diseases it is being developed in. I used data from Hanson Wade’s Beacon Adoptive Cell Therapy database to put the graphic together. Rather than go with the typical “stack chart” format for landscapes here, I wanted to showcase a view that allows you to see the breadth of indications these therapies are being developed in as well as how far along (i.e. what phase) each is in for each disease.
For this exercise, I focused on company-sponsored (i.e. non-academic) programs that are already in the clinic or have had INDs cleared to begin clinical studies. Looking at the pre-clinical landscape, it was a bit difficult to determine indication prioritization for many programs, as seemingly most are listed as going after “autoimmune disease” or “inflammation” broadly, so I felt it best to leave them out for this first iteration. I excluded programs that were based on non-immune cell types (e.g. like stem cells) and excluded cell therapy vaccines (typically dendritic cells). I also omitted certain autoimmune diseases here like Type 1 Diabetes (which is its own world entirely that I’d like to cover in a future post) as well as GvHD.
Notably, new updates since the last version of the map are demarcated in two ways:
New programs/companies that have entered the landscape are highlighted in yellow
Updates to program statuses in individual indications are denoted by an all-black boarder (e.g. if a program in lupus moves from IND to P1/2 the box for P1/2 will be bordered black)