Some signs of life for new IL-2 variants
The annual AACR meeting was held last week. Below I highlight a couple of data readouts from two players in the once-hot IL-2 variant space
Hello reader! Your sponsorship could go right here at the very top of the next Big Pharma Sharma post. A sponsored post is 100% accessible to all subscriber levels and gets your brand, product, or service in front of my audience of BioPharma Industry decision-makers. If you or your company is interested in becoming a Big Pharma Sharma sponsor, please reach out to me here or on my socials. Click the button below to learn how you can sponsor the next edition of Big Pharma Sharma.
There was a ton of intriguing clinical and preclinical data unveiled at last week's annual AACR meeting. Given the novelty of the molecules, mechanisms of action, and combinations presented, this conference is always a challenge to synthesize completely.
For today's post, I chose to spotlight one specific area – IL-2 variants – and dive into a couple of compelling data readouts from emerging players in this space.
The IL-2 Conundrum
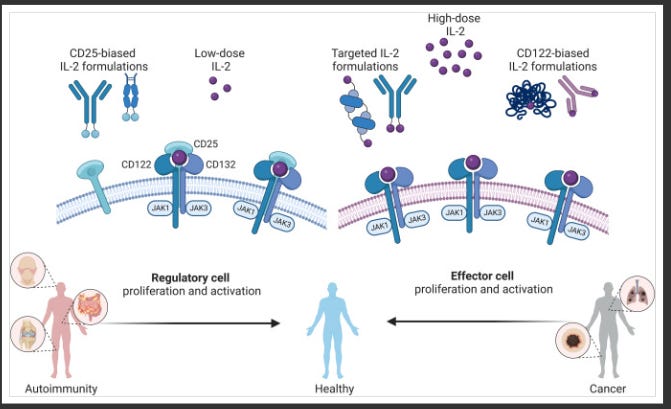
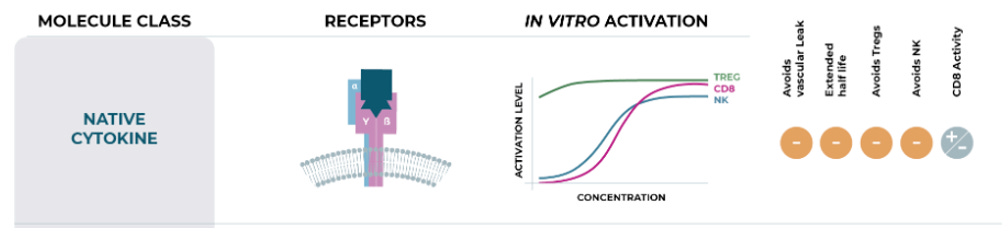
Many companies have tried and failed to harness IL-2 as an effective oncology treatment, most notably Nektar, whose once-promising bempegaldesleukin (pegylated IL-2) program catastrophically flamed out in a phase 3 study in first-line melanoma. The primary stumbling block with leveraging IL-2 in an oncology context is its broad activation profile, which includes the intended stimulation of effector T cells, but also the undesirable activation of regulatory T cells (Tregs), largely driven by engagement of the alpha subunit of the IL-2 receptor (aka CD25). This indiscriminate activation can precipitate potentially lethal side effects while simultaneously undermining efficacy if Tregs become overstimulated.
These limitations have spawned a multitude of players tinkering with modifications to the IL-2 molecule, generating IL-2 variants, in an effort to skew its mechanism of action toward preferentially activating effector T cells while limiting Treg stimulation. Two companies - Medicenna and Synthekine - advancing IL-2 variants as their lead programs, presented early clinical data at AACR, each showcasing a distinct spin on engineering the IL-2 molecule.
Synthekine and Medicenna: Divergent IL-2 Variant Designs Yield Intriguing Early Results
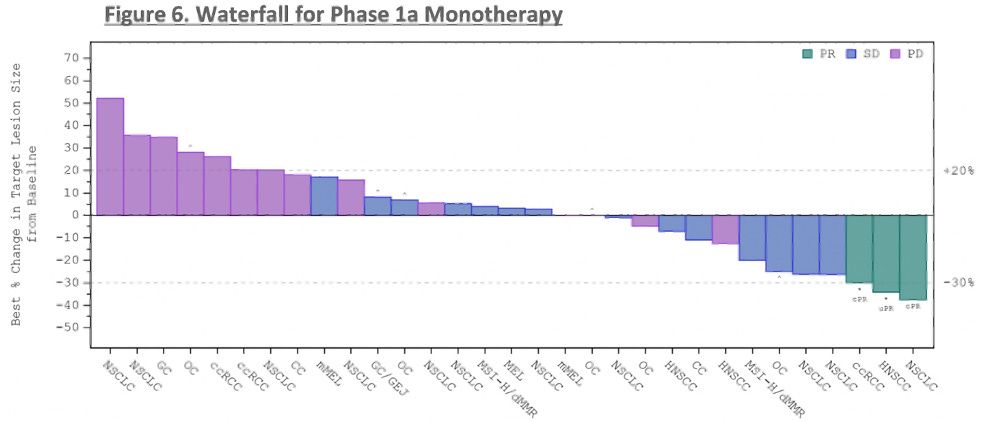
Synthekine presented first clinical data from its lead program, STK-012, a first-in-class alpha/beta-IL-2R biased partial agonist. The molecule has been designed to preferentially bind the high-affinity IL-2Rs while reducing binding to the IL-2R gamma chain, with Synthekine's hypothesis being that this design selectively activates antigen-primed T cells. Across a wide range of heavily pre-treated solid tumor patients, STK-012 monotherapy delivered 3 partial responses (PRs) in renal cell carcinoma, head and neck squamous cell carcinoma, and non-small cell lung cancer, along with 12 instances of stable disease. Mechanistically, Synthekine demonstrated STK-012's selectivity for CD25-expressing cells, dose-dependent increases in IFN-gamma secretion, and limited expansion of off-target cell subsets like NK cells and Tregs.
While these initial three responses represent an encouraging early sign in traditionally immunotherapy-sensitive tumor types, the pivotal path for STK-012, like most IL-2 variant programs, will likely involve combination regimens with checkpoint inhibitors. Nonetheless, demonstrating monotherapy responses helps allay potential efficacy concerns ahead of future combination-focused studies. Safety was relatively clean aside from some grade 3 GI toxicities at the highest 3mg dose level and one grade 4 anaphylactic reaction that rapidly resolved.
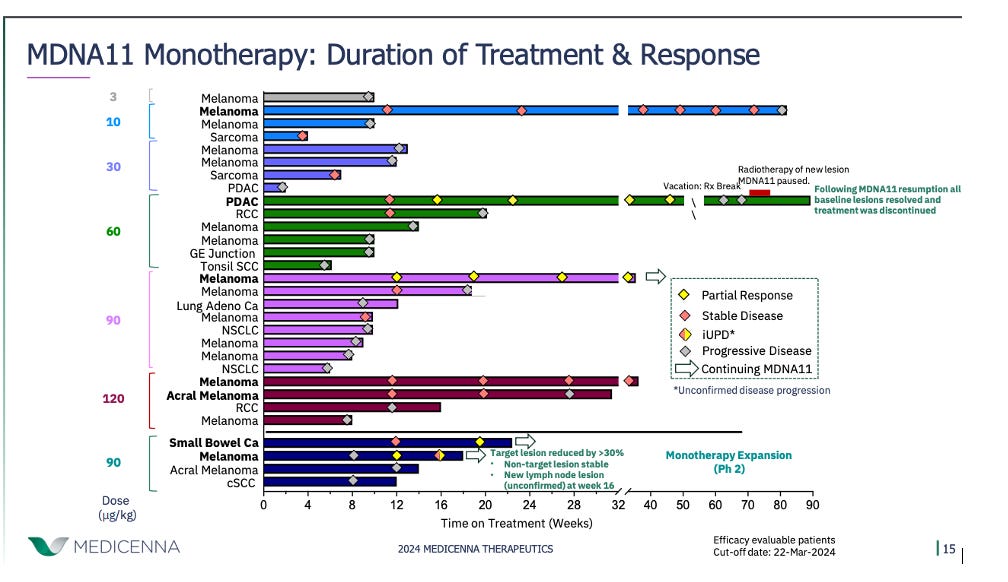
Medicenna also presented updated phase 1 data for its IL-2 variant, MDNA11, in 38 patients. MDNA11 is engineered as a "beta-enhanced, not-alpha" IL-2 construct fused to albumin to extend its half-life. Medicenna believes this design enables MDNA11 to selectively activate CD8+ T cells and NK cells while minimally engaging Tregs. The company reported 4 PRs across difficult-to-treat tumor types like pancreatic ductal adenocarcinoma, small bowel cancer, and melanoma. Medicenna somewhat clumsily characterized this as a 28.6% response rate in "phase 2 eligible" patients receiving doses ≥60 mcg/kg, though a more conservative calculation pegs the ORR closer to 18%.