Rethinking the FDA, Innovation, and the Regulatory State: Parsing Vivek and Balaji’s Vision
An examination of Vivek Ramaswamy's latest conversation with Balaji Srinivasan on the role of the FDA and their vision for dismantling it and the rest of the "regulatory state"
When people like Vivek Ramaswamy and Balaji Srinivasan sit down to talk, you know it’s going to be thought-provoking. Their recent discussion took aim at the FDA and the broader “regulatory state”, suggesting that these institutions are more of a hindrance than a help. They envision a future where innovation isn’t hampered by bureaucracy, where the FDA’s power is drastically reduced or even replaced by new systems.
It’s a compelling vision, but it misses an important point: we operate within the framework of the current system, and starting from scratch isn’t realistic. Instead of focusing on tearing down what exists, we need to think about how to build bridges to meaningful change, recognizing the complexities of the system while aiming for a future that’s both optimistic and achievable.
Note: a quick aside for some background on the two personalities of interest here in case you haven’t heard of them before:
Vivek Ramaswamy is best known in biopharma circles as the founder of Roivant Sciences, the company known for in-licensing overlooked and abandoned big pharma assets on the cheap, spinning them out into standalone companies, and accelerating their development into commercializable drugs. Outside of biopharma he is probably best known for being a regular talking head on conservative news programs, his brief run for Republican party’s presidential ticket, and now being somewhat of an acolyte of Donald Trump.
Balaji Srinivasan is best known for his past roles as a General Partner at a16z, former CTO of crypto platform Coinbase, and overall crypto evangelist. In the interview, he mentions some earlier entrepreneurial efforts in biotech, but I would say he is most well-known in tech circles. On Vivek’s podcast, called “The Truth”, the topic of dismantling the FDA is a major focus, as Balaji was rumored to have been offered a high-level position at the agency in the Trump administration.
Guess what, reader? Your sponsorship could go right here at the very top of the next Big Pharma Sharma post.
A sponsored post is 100% accessible to all subscriber levels and gets your brand, product, or service in front of my audience of BioPharma Industry decision-makers.
If you or your company is interested in becoming a Big Pharma Sharma sponsor, please reach out to me here or on my socials. Click the button below to learn how you can sponsor the next edition of Big Pharma Sharma.
The FDA: Innovation’s Friend or Foe?
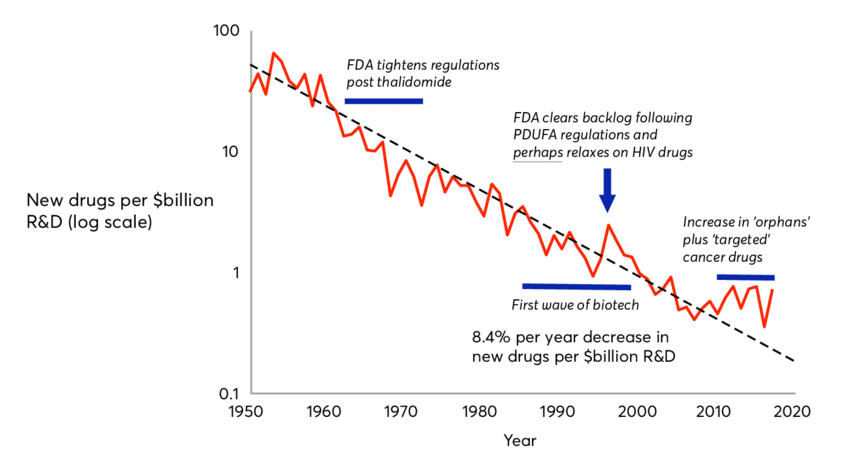
Vivek and Balaji argue that the FDA, with its complex regulations, often acts more as a roadblock than a safeguard. They cite Eroom’s Law—a concept that suggests the cost of drug development has soared, partly due to the FDA’s increasing demands. It’s a compelling argument, especially when you consider the challenges of bringing new drugs to market.
Moreover, I’m not sure that the increased regulatory hurdles are the primary driver of drug development costs. Medicine is shifting towards to more complex molecules, like DNA/RNA, cell, and gene-based therapies. These classes of therapies, in many cases, offer much greater therapeutic potential, but require more specialized and less scalable (at least currently) manufacturing processes that results in higher COGS. Part of the increasing costs too are likely due to being victims of our own success. As medicines get better in a particular disease the bar new medicines need to meet or exceed to warrant viable commercial opportunity also goes up.
While the FDA’s caution can feel like a hurdle, especially for those working on the cutting edge of biotech, removing it entirely would be risky. It’s like suggesting we ditch seatbelts because they take a few extra seconds to buckle up—sure, it might save time, but the potential cost is far too high. Instead of viewing the FDA as the enemy, we should be discussing how to evolve it into an agency that better balances necessary caution with the speed needed for innovation. The goal shouldn’t be to tear down, but to build something that works better for everyone.
The Regulatory State: A Necessary Evil?
Vivek and Balaji expand their critique to the entire regulatory state, arguing that it often stands in the way of progress. This sentiment is especially resonant in tech circles, where speed and disruption are highly valued. The risk-reward of “move fast and break things” works ok when you’re dealing with ones and zeros, but can be potentially disastrous and net harmful when those ones and zeroes are actual human lives in the context of drug development.
This perspective overlooks the essential function of regulatory bodies like the FDA, SEC, and FAA. These agencies aren’t just bureaucratic hurdles—they’re there to prevent systemic failures that could have catastrophic consequences. Yes, they can be slow to act, reticent to adapt, misaligned with innovation, and even at times overreach with their power, but their role is to ensure safety and fairness in industries that impact millions of lives.
The challenge isn’t to tear down the regulatory state but to make it more adaptable, more responsive to the pace of modern innovation. The future we’re aiming for shouldn’t be about destruction—it should be about thoughtful evolution, recognizing where we are today and how we can build something better. The narrative needs to shift from fear and negativity to one that embraces the potential for positive change.
Building Alternatives: The Road Less Traveled
Vivek and Balaji suggest that instead of reforming the system, we should build alternatives—pointing to examples like Bitcoin, SpaceX, and medical tourism, where innovation has flourished outside traditional regulatory frameworks. It’s a bold idea, and one that has worked in certain contexts. Although, I am not totally sure how it would work with the FDA. Would we just create an alternative drug approval body?
However, the idea that we can bypass regulation entirely, particularly in sectors like healthcare, is overly simplistic. Healthcare isn’t just another industry; it’s one where the stakes are incredibly high, and the consequences of failure are measured in lives, not just dollars. While creating alternatives is valuable, these alternatives still need to operate within a framework that ensures safety and effectiveness.
Rather than discarding the current system, the focus should be on how we can create and integrate these alternatives in a way that respects and enhances existing regulatory frameworks. The vision for the future shouldn’t be about abandoning the present but about finding practical, optimistic ways to evolve it. It’s not just about the destination—it’s about charting a path that gets us there in a realistic and achievable way.
Reform, Not Revolution: A Path Forward
The idea of a world where innovation isn’t stifled by bureaucracy is undeniably appealing. Who wouldn’t want faster drug approvals, more efficient processes, and fewer barriers to progress? But before we rush to dismantle the regulatory state, we need to consider a more balanced approach—one that favors thoughtful reform over wholesale revolution.
The FDA plays a crucial role in ensuring that the drugs we take are safe and effective. The same goes for other regulatory bodies—they exist because history has shown us the dangers of unchecked progress. Rather than tearing down these institutions, our focus should be on modernizing them, making them more agile, and better suited to the demands of the 21st century.
Imagine a regulatory system that’s faster, more flexible, and better attuned to the needs of innovators. This isn’t an impossible dream—it’s a goal that requires smart, nuanced reform, not reckless destruction. The future we want is one where change is not only possible but also practical, rooted in a deep understanding of where we are today and an optimistic vision of what we can achieve together.
Conclusion: Balancing Innovation and Regulation
Vivek and Balaji have sparked an important conversation about the future of regulation and innovation. Their critiques of the FDA and the regulatory state are sharp, and their call for alternatives is compelling. But as we look toward the future, the goal shouldn’t be to pit innovation against regulation—it should be to find a way for them to coexist and complement each other.
The real challenge ahead isn’t choosing between freedom and oversight—it’s figuring out how to have both. And if we can strike that balance, we’ll be far better equipped to meet the challenges of tomorrow. The inspiring, paradigm-shifting future we need has to be one that is shared in a way that’s doable, connecting the present with the possibilities of what lies ahead.