Mini ASCO 2024 Preview: An Emerging Battle in Frontline Melanoma
A little mini preview ahead of ASCO later this week to prime your minds and raise your awareness to a potentially impactful set of readouts
Guess what, reader? Your sponsorship could go right here at the very top of the next Big Pharma Sharma post.
A sponsored post is 100% accessible to all subscriber levels and gets your brand, product, or service in front of my audience of BioPharma Industry decision-makers.
If you or your company is interested in becoming a Big Pharma Sharma sponsor, please reach out to me here or on my socials. Click the button below to learn how you can sponsor the next edition of Big Pharma Sharma.
ASCO 2024 is poised to be a pivotal event for melanoma therapies, with several innovative approaches set to report early data. Let's explore the most exciting data and what it could mean for the future of melanoma treatment.
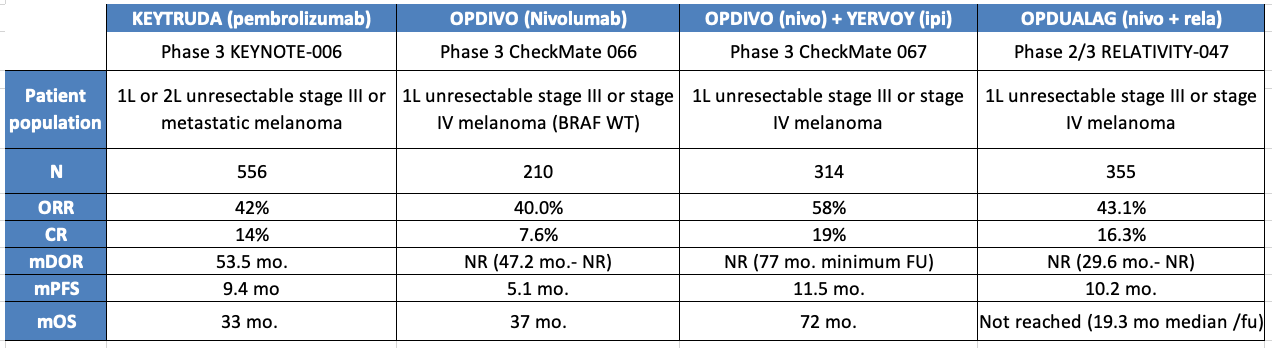
First-Line Melanoma: A Shifting Landscape
The current first-line (1L) treatment for melanoma includes PD-1 monotherapy (KEYTRUDA or OPDIVO) typically for older patients with PD-L1 expression and the Nivo+Ipi combo (PD-1 + CTLA-4) for younger, healthier patients or those with aggressive disease. The arrival of OPDUALAG (PD-1 + LAG-3 FDC) is changing this landscape with its higher efficacy and lower toxicity compared to Nivo+Ipi, seemingly pulling patients from both segments.
Recently, the approval of Iovance’s AMTAGVI (lifileucel) has introduced a new contender. Initially approved for use after PD-1 therapy, Iovance is now aiming to prove its effectiveness in the 1L setting through the P3 TILVANCE study, which is examining AMTAGVI in combination with KEYTRUDA. Early data from the IOV-COM-202 study in 1L melanoma, to be presented at ASCO, offers a glimpse into how this combination may stack up vs. the current standard of care.
Join your friends and colleagues who are getting BioPharma insider analyses and insights in their inbox each week. It’s less than $10/month and you can probably even expense it!