Flipping the Script on Failed Immuno-Oncology Targets: The Case for “Inverse-I/O” in Autoimmune Diseases
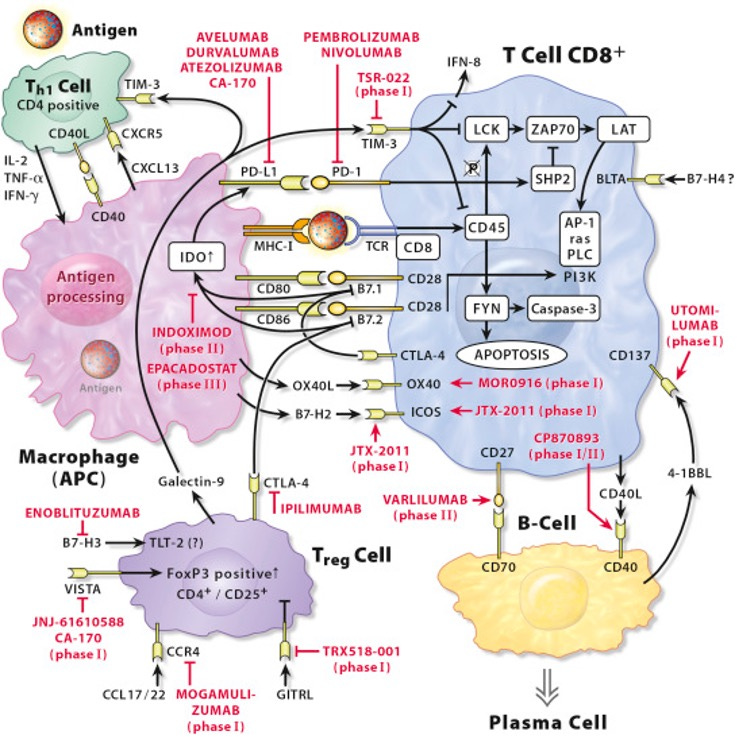
Since the success of PD-(L)1 therapies, immuno-oncology (I/O) has been a hotbed of activity, with companies scrambling to find the next blockbuster target. But for every success story, there’s a long list of targets that just didn’t work out in oncology, like IDO, 4-1BBL, OX40, and CD40. While these targets didn’t pan out in cancer, they may hold untapped potential if we “flip the script” and explore their inverse mechanisms—targeting them not to stimulate, but to suppress immune activity in autoimmune diseases.
The Cancer-Autoimmune Axis
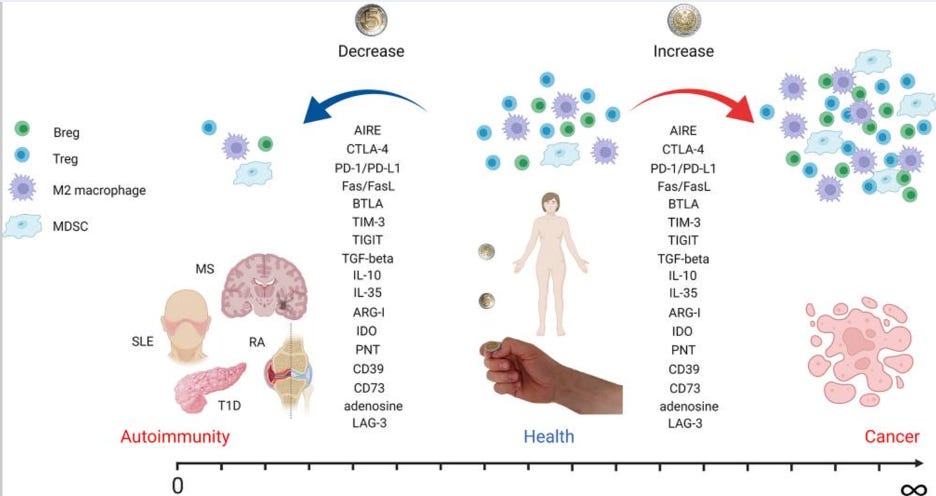
Cancer and autoimmune diseases are almost opposites in terms of immune activity. In cancer, the immune system is underactive, and drugs like PD-1 inhibitors work by removing the brakes on T-cell activity to help the immune system attack cancer. In autoimmune diseases, the immune system is overactive, so the goal is to rein in immune responses, often by blocking pro-inflammatory pathways or activating anti-inflammatory ones. This dynamic suggests that previously unsuccessful oncology targets could be re-engineered to shut down, rather than stimulate, the immune response—providing a potential breakthrough in autoimmune and inflammatory diseases.
Inverse-I/O in Action: CD40L and OX40L
The table below illustrates two promising “inverse-I/O” examples: CD40L and OX40L. Both targets failed in the oncology context but are now gaining traction in autoimmune diseases, with multiple Phase 3 trials in progress. These trials highlight how leveraging the inverse mechanisms of failed I/O targets may open up new therapeutic pathways in autoimmune and other diseases.