ASH Part 2: Anito-cel is off to the races, but will it be fast enough to catch-up to Cilta-cel?
Arcellx presented Phase 2 pivotal iMMagine-1 data for anito-cel, their BCMA-targeted CAR-T for 5L+ relapsed/refractory multiple myeloma (RRMM), at ASH 2024. This was long anticipated data, as P1 anito-cel data (which was also updated at ASH) painted the picture of a real contender to market leader CARVYKTI (cilta-cel) marketed by J&J and Legend, which is already approved in the 5L+ setting, and as of earlier this year also in the much broader 2L+ setting.
There was a time where BMS’ ABECMA was neck-and-neck with CARVYKTI, but as pivotal data readouts demonstrated a clear efficacy advantage with CARVYKTI and quarterly sales numbers continued to come in, it became clear that CARVYKTI was the sole dominant force in the BCMA CAR-T space.
Enter anito-cel, which at ASH provided a first look at what its on-label product profile could look like and is trying to make a case that they have a best-in-class profile, relative to CARVYKTI.
Key highlights include (note: slides from Arcellx investor call with data can be found here):
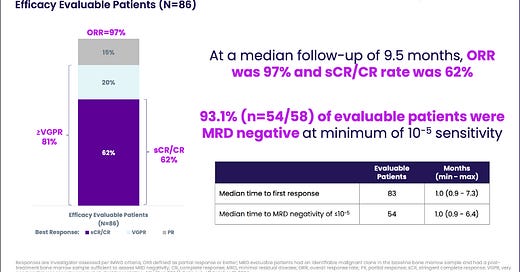
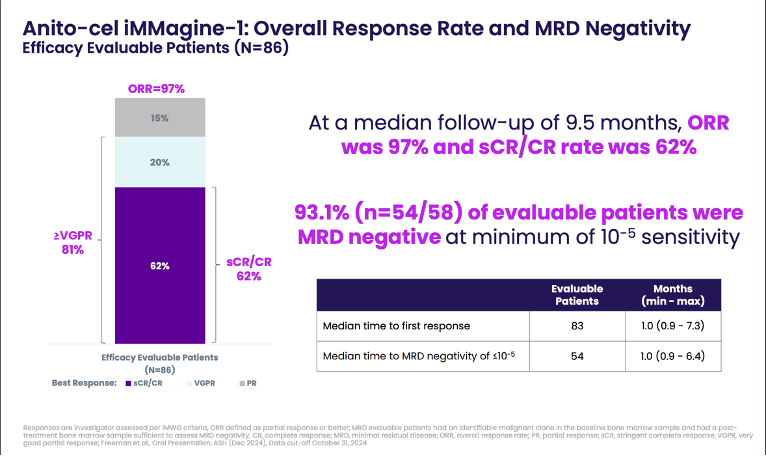
Efficacy: Among 86 efficacy-evaluable patients (median follow-up 9.5 months), the overall response rate (ORR) was an impressive 97%, with 62% achieving complete response or stringent complete response (CR/sCR). Minimal residual disease (MRD) negativity was achieved in 93.1% of tested patients at a sensitivity of 10-5
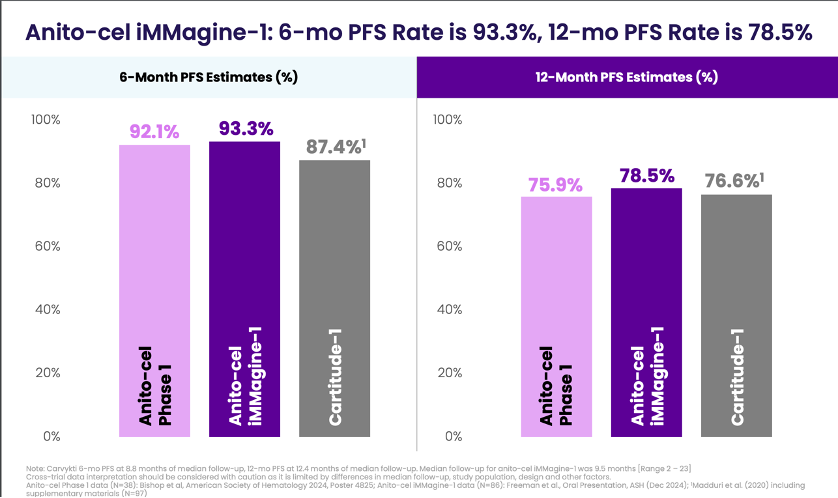
Durability: Median progression-free survival (PFS) and overall survival (OS) were not reached, but 6-month PFS and OS rates were 93.3% and 96.5%, respectively, and 12-month rates were 78.5% and 96.5%
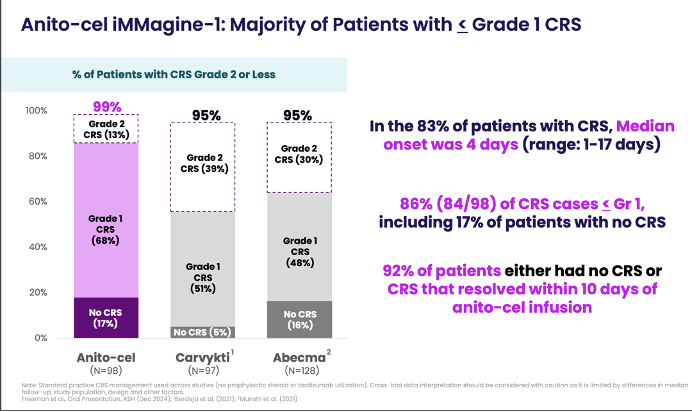
Safety: The safety profile continues to look favorable with no cases of delayed neurotoxicities, including Parkinsonism or Guillain-Barré syndrome, observed across more than 150 patients treated. CRS was largely low grade (86% ≤Grade 1), with a median onset of 4 days. ICANS was observed in 9% of patients, with only 1% experiencing Grade 3. Most cases resolved without long-term effects.
Notably there was on case of lethal CRS (Grade 5) in an older patient with comorbidities and rapidly progressive disease at baseline who did not receive bridging therapy. Two other deaths on-study were deemed to be unrelated to anito-cel.
These results, along with the Phase 1 data showing a median PFS of 30.2 months at 38.1 months follow-up, help add credence to the story Arcellx is trying to tell about anito-cel as being at least equivalent to CARVYKTI in efficacy, but with a much cleaner safety profile.
If it’s not evident from the slide images I posted above, Arcellx doesn’t seem to think too highly of Legend/CARVYKTI. Arcellx also decided to release updated anito-cel data late in the evening while Legend was holding an analyst meeting at the same time
Side note: I’ve never seen a corporate slide deck compare their own data so deliberately to and negatively of a market leader. Frankly, I love it, and I think more companies should use this more direct form of messaging vs. the cookie-cutter talking around what they are really trying to say aided by consulting and banking lingo.
Over the course of the conference, we learned that the feeling is mutual, with Legend referring to anito-cel as a “me too drug” in an interview with Brad Loncar on BiotechTV. The funniest thing about this comment was that it came right at the end of the interview unprompted from Legend’s CEO. Got to love when two biotechs are passive aggressively beefing. Is Arcellx living in Legend’s head rent-free?