ASCO 2024 Temperature Check – Part One
Below is part one of my ASCO review where I do a "temperature check" and qualify what's hot, tepid, and cold coming out of the conference.
Guess what, reader? Your sponsorship could go right here at the very top of the next Big Pharma Sharma post.
A sponsored post is 100% accessible to all subscriber levels and gets your brand, product, or service in front of my audience of BioPharma Industry decision-makers.
If you or your company is interested in becoming a Big Pharma Sharma sponsor, please reach out to me here or on my socials. Click the button below to learn how you can sponsor the next edition of Big Pharma Sharma.
We just finished up another grueling ASCO with very interesting data readouts that could shift the outlook for several companies, markets, and modalities across the world of oncology. To be honest, I can’t remember an ASCO of late that was a total dud. Expectations running into this ASCO from the broader zeitgeist were that this year’s rendition wouldn’t be as interesting as prior years, but I didn’t find that to be the case at all. Par for the course, I found myself overwhelmed by how many potentially shifting data readouts there were at this year’s ASCO and having a hard time narrowing these down into what I felt was most impactful coming out of the conference.
For past conferences, I put together a winners and losers report. I was thinking of doing the same for ASCO but decided to go with a slight reframe. After all, science is hard work, and failure is part of the process. We’re all trying to do our best to get new medicines approved for patients who need them the most.
Below is my ASCO summary in the form of a “temperature check”. Which companies, modalities, molecules, etc. outlooks have risen and become hot? Which have fallen out of favor and gone cold? And which have started to feel like a tepid bubble bath? Just for an added bit of fun, each section will have a representative song to go with it.
This is projecting to be quite a meaty topic, so I decided to split this one up into two posts. Below is part one.
Hot: Nelly, Hot in Herre
I/O Bispecifics: They’re back!
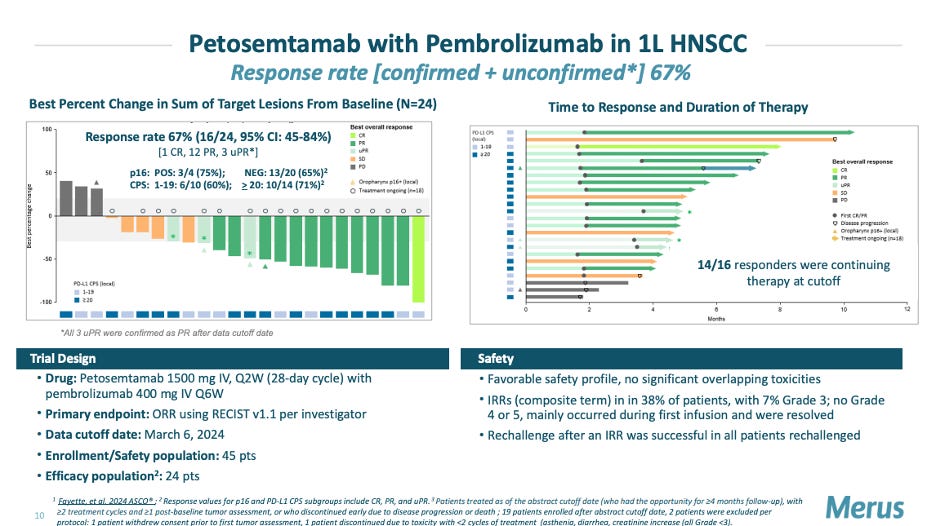
ASCO saw a surprise revival of the once promising I/O bispecifics space. New data from Merus’ petosemtamab (LGR5xEGFR) in 1L HNSCC, Akeso/Summit’s ivonescimab (PD-1xVEGF) in 1L NSCLC, and Genmab/Biontech’s acasunlimab (PD-L1x4-1BB) in 2L NSCLC were major highlights that may draw more eyes back into this space.
Peto showed a 67% ORR (13/24 patients) in combo with pembrolizumab, which compares favorably to cetuximab+ PD-1, which has an ORR around 45% in 1L HNSCC. The company intends to initiate a P3 study in 2L/3L HNSCC as monotherapy vs chemotherapy or cetuximab. Emerging data in this setting has shown a 37% ORR and based off the ASCO readout, we should not be surprised if plans for larger P3 study in 1L setting get announced sooner rather than later. However, competition remains steadily fierce in this setting, with players like Genmab reporting data of TIVDAK (Tissue facotr ADC) in 2L/3L HNSCC, at the conference, generating a 40% ORR.