Safety signals in Lyell’s ROR-1 CAR-T data put key strategic decisions in focus
Below I take a look at first clinical data from Lyell's ROR-1 CAR-T program, LYL797, and discuss potential strategic implications
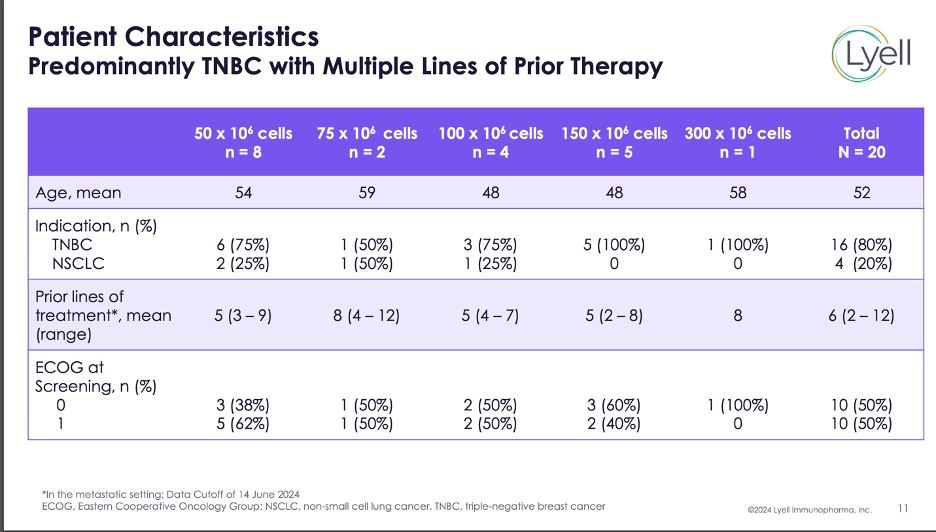
Last week we finally saw clinical data from Lyell Immunopharma’s lead next-gen CAR-T program, called LYL797 in solid tumors (TNBC and NSCLC).
LYL797 is a ROR-1 CAR-T that employs overexpression of c-Jun and epigenetic reprogramming (Lyell’s Epi-R technology) to combat exhaustion and confer durable stemness on the CAR-T product. The company, which at one point had over $1B in cash on its balance sheet, raised bags upon bags of money based off the promise of these T-cell reprogramming technologies. I’ve been following the company for a while and conceptually always found these technologies to be unique and interesting ways of improving T-cell fitness/exhaustion-resistance. Over time the company’s share price steadily tumbled downwards, IPO’ing at a valuation of ~$4B and landing at ~$370M where it sits today. I suppose there is only a limited amount of time you can highlight all the advanced pre-clinical engineering work you’re doing before people start saying “yah that’s cool, but let’s see some clinical data.” That day final came on June 26th.
In the wake of AstraZeneca’s tremendous CAR-T data in HCC, presented at ASCO, LYL797’s data in TNBC and NSCLC was not so impressive, raising significant safety concerns, notably one case of fatal pneumonitis in a patient with TNBC and lung metastases. Patients (n=20) were treated across five different dose levels, with all patients being heavily pre-treated, and most patients being TNBC patients.
Two patients achieved a partial response durable out to 90 days (both at the 150M cell dose) and the one patient treated at the highest dose (300M) had a stable disease. It’s great to see responses obviously, but its clear more data are needed to truly assess whether the exhaustion and stemness benefits conferred by Lyell’s core technologies are translating into a clinically significant benefit. The company also did not provide any guidance on correlation between ROR1 expression and efficacy, saying it was too early at this juncture.