Can Roche’s COLUMVI threaten the CAR-T renaissance in DLBCL?
Below I examine Roche’s P3 STARGLO study of Columvi (CD20xCD3 TCE) + chemo in 2L DLBCL and examine if/how these new results could threaten CD19 CAR-Ts in NHL
I know we just got done with ASCO, which means that EHA is right around the corner. Regular attendees of both conferences can attest to the fact that EHA can often be filled with encore presentations from the preceding ASCO meeting, especially in years where ICML Lugano also take place. However, this year we saw some new and interesting data from the swath of heme/onc players, most notably Roche – the proverbial Silverback in the heme/onc field.
Roche has been looking to shape life after Rituximab for some time now and has built a portfolio of biologics in heme malignancies to take the place of its one prized portfolio cornerstone.
Obviously given the indications the rituximab franchise encompassed, the advent of CD19 CAR-T therapy has revolutionized standards of care across Roche’s bread & butter indications, like DLBCL, FL, and CLL. DLBCL is where CAR-Ts have done the most damage, offering patients a curative one-time treatment option in the 2L (transplant eligible) and 3L+ settings. Thus far, the major competitor to CAR-Ts have been themselves, with the multitude of approved cell therapy products all competing for the same resources at the subset of treatment centers accredited to perform CAR-T infusions. In the broader 2L setting, CAR-Ts have largely penetrated the transplant-eligible segment, offering younger/fitter patients a more powerful and definitive curative option than autologous stem-cell transplant. BMS’ BREYANZI (CD19 CAR-T) is also approved in the transplant-ineligible setting, but anecdotally uptake in this segment has seemed to be limited, as the definition of “transplant-eligible” has slowly changed to be equivocated to some degree with “CAR-T-eligible”.
Interested in sponsoring the next edition of Big Pharma Sharma?
A sponsored post is 100% accessible to all subscriber levels and gets your brand, product, or service in front of my audience of BioPharma Industry decision-makers.
If you or your company is interested in becoming a Big Pharma Sharma sponsor, please reach out to me at shivu@bigpharmasharma.com to learn more. You can check out a recent post by one of our sponsors here to get a sense of what it’s like.
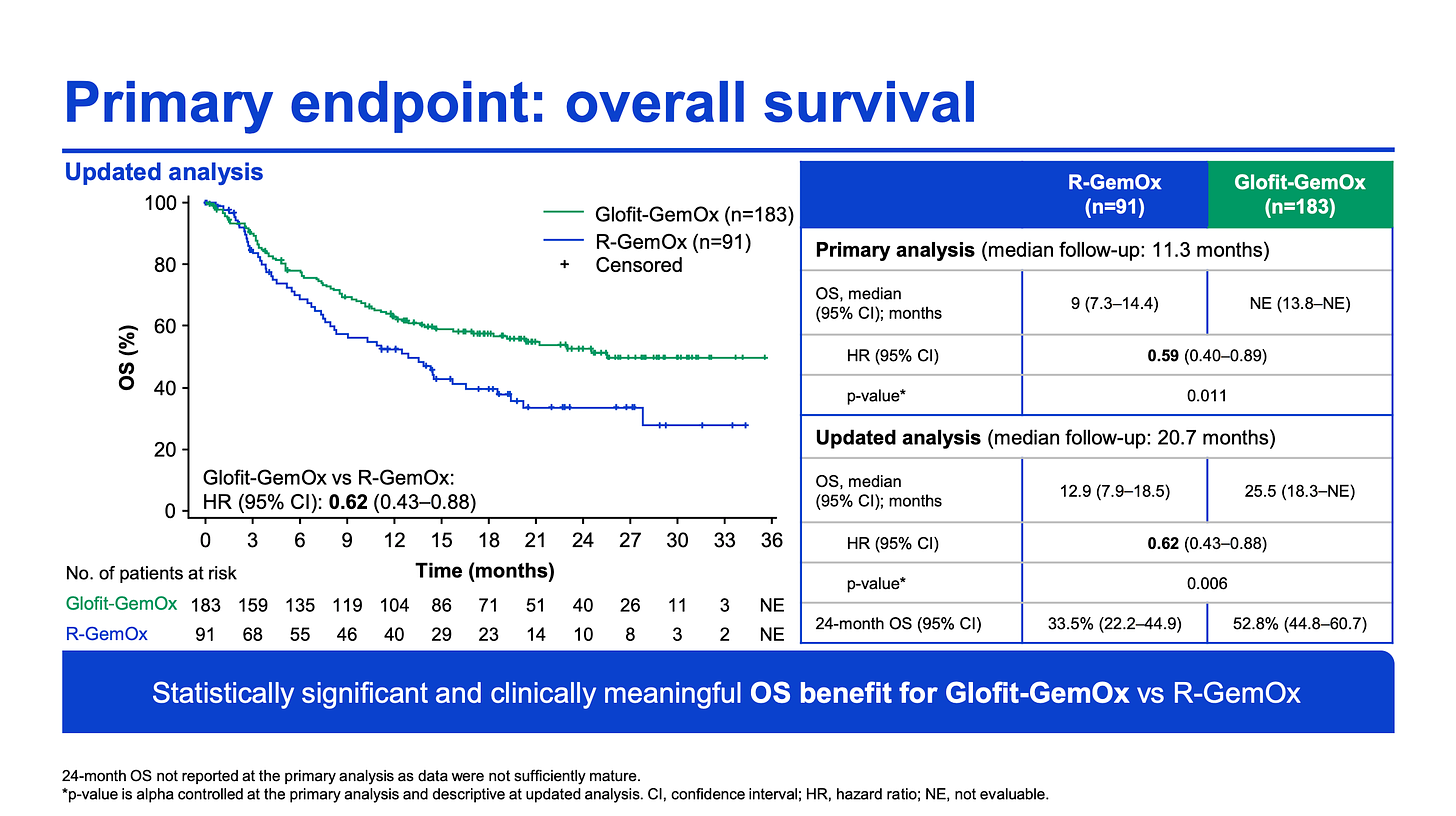
So now in comes Roche with their brand new STARGLO data. The P3 trial studied COLUMVI (glofitamab, glofi) + GemOx (n=183) vs. Rituximab (R) + GemOx (n=91) in patients with 2L transplant ineligible DLBCL. Notably the glofi regimen was a fixed treatment duration, with patients receiving the full combo for eight 21-day cycles and transitioning to a glofi monotherapy for a subsequent four cycles.
And the results? Efficacy-wise the glofi combo knocked it out of the park, nearly doubling the OS (25.5 vs. 12.9 months; HR:0.62) and quadrupling the PFS (12.1 vs. 3.3 months; HR:0.37). Response rates were significantly higher in the combination arm as well, with glofi demonstrating a 68% ORR (59% CR) vs. R-GemOx’s 41% ORR (25% CR).
If we use BREYANZI’s transplant-ineligible label as a comparator, the glofi-GemOx combo looks comparable. BREYANZI’s response rate was 80% (54% CR), mPFS was 9 months, and mOS not reached at 23 months of follow-up in a much smaller (n=61) study.
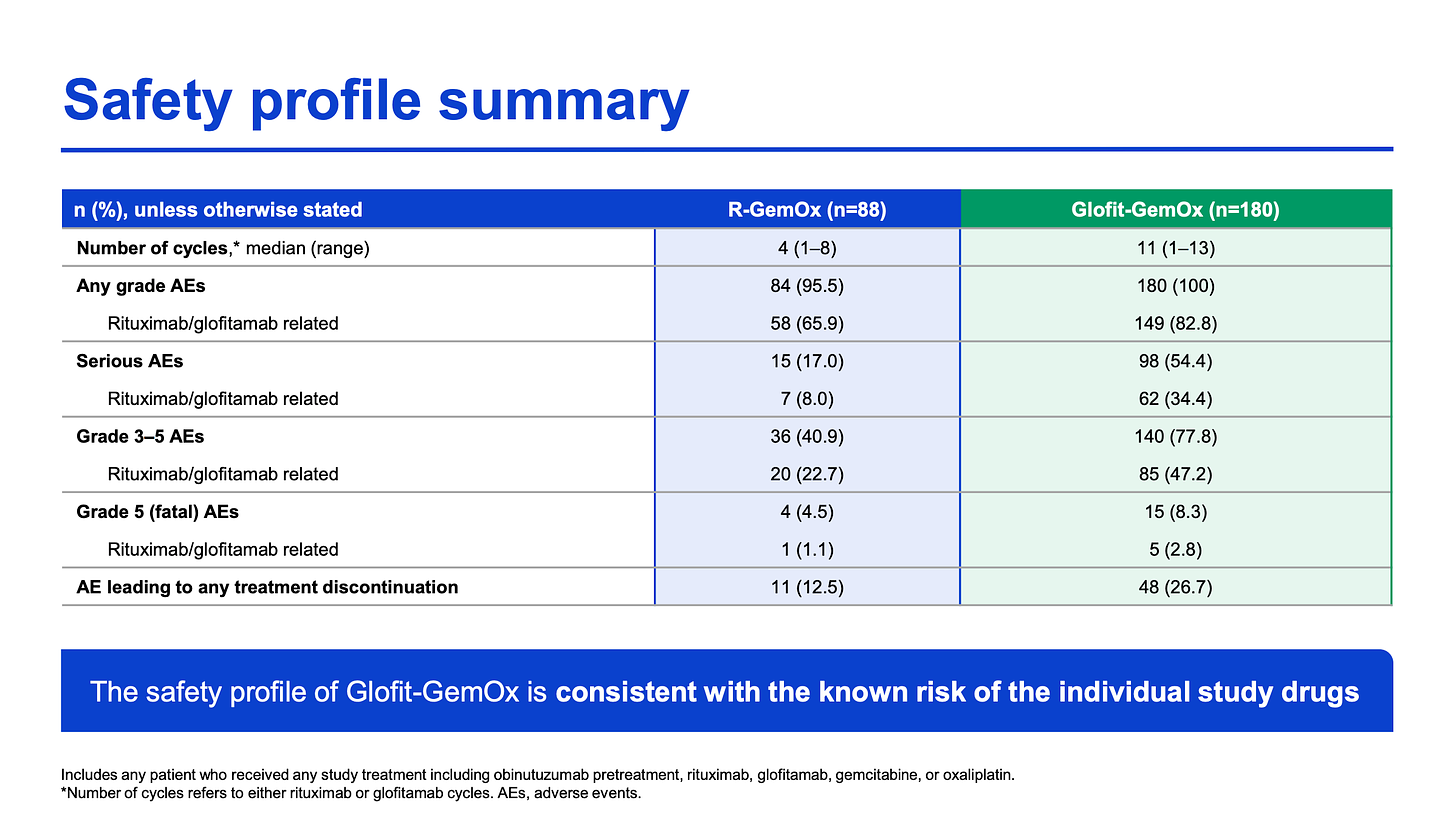
From a safety perspective, the glofi combo did demonstrate increased toxicity relative to R-GemOx, with a notable a near doubling in G3+ infections, which could pose an issue for some patients. But still, in comparison to the perceived toxicity that comes with CAR-T therapy, the glofi combo looks favorable.