BBT: Replimune (REPL) Looking to Restore the Promise of Oncolytic Viruses in Skin Cancer and Beyond
Below is the second installment of my “BioPharma Buyout Targets” (BBT) series where I take a look at intriguing acquisition targets for Big BioPharma players. Today we will be focusing on Replimune.
Executive Summary
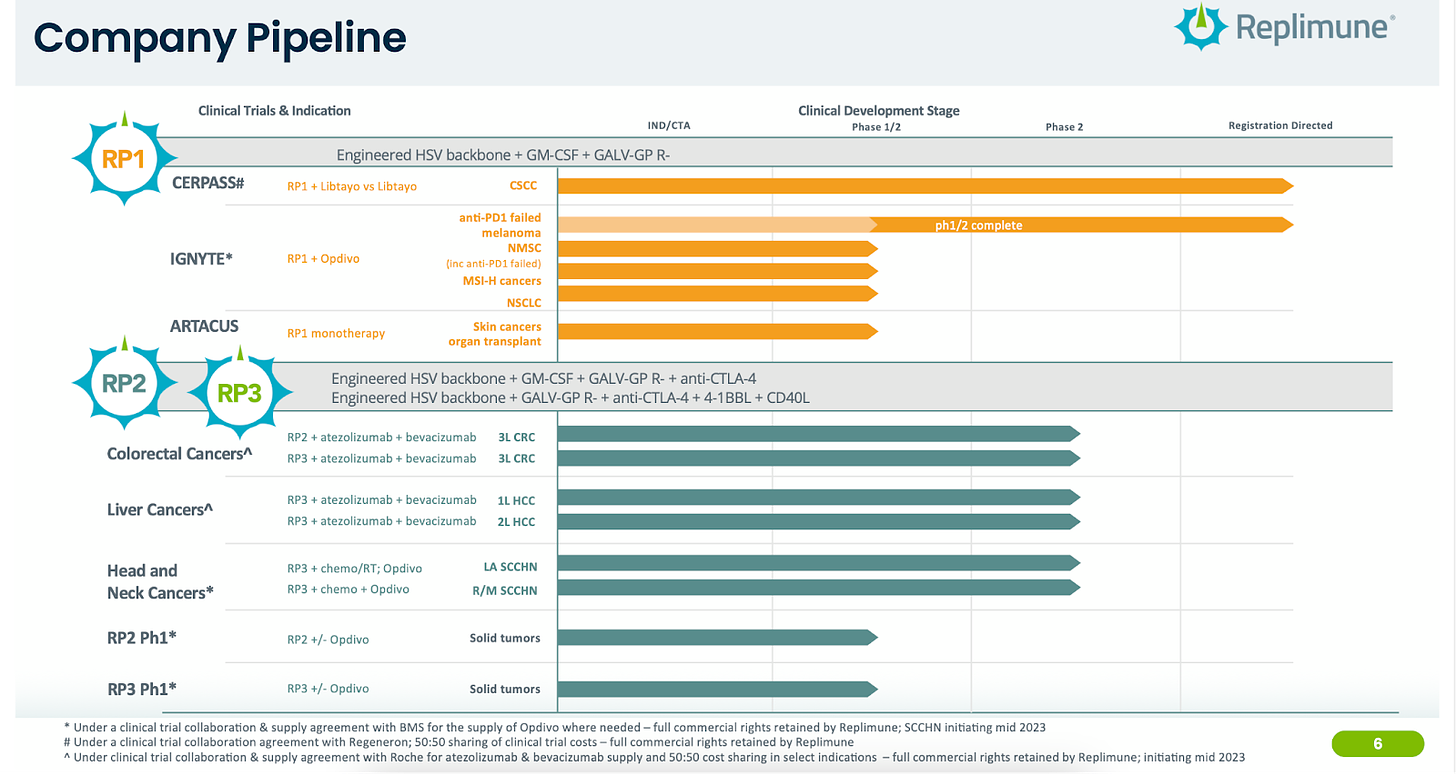
Replimune is a late-stage company hoping to deliver on the once highly touted potential of oncolytic viruses. Its lead asset, RP1 could meaningfully improve the standard of care in combination with PD-1 mAbs in both CSCC and the much larger 2L+ melanoma setting. Pivotal data readouts in these two indications are set to be released over the next three to six months, forming the basis of a build towards a significant skin cancer franchise across lines of therapy in melanoma, CSCC, and other non-melanoma skin cancers. RP1 appears to be able to meaningfully increase CR rates in CSCC in combination with PD-1, while in melanoma, appears to have a profile at least in line with TIL therapies, but with off-the-shelf convenience and a wider prescriber base. Next-generation versions of RP1 (RP2 and RP3) are mid-stage assets that offer interesting upside in other lesion-accessible solid tumors (HCC, SCCH, and CRC) and could meaningfully expand the total addressable market of Replimune’s OV platform. Acquisition of Replimune could prove to be a strong strategic fit for I/O skin cancer players like Regeneron or a mid-size company looking to make a name for itself in this space, like Incyte, among others.
Platform & Pipeline Overview
Platform/Company Focus
Replimmune is an oncoloytic virus (OV) company. Oncolytic viruses were all the rage about 5-10 years ago. The first approved OV therapy belongs to Amgen (via its acquisition of BioVex for $1b in 2011) and is called IMLYGIC (aka T-VEC). To put it plainly, IMLYGIC has not been a commercial success, registering sales too small for Amgen to even report in their quarterly filings. Replimune has put some new twists on T-VEC and created a OV platform that it believes can actually deliver differentiating and therapeutically meaningful efficacy to patients suffering from skin cancers.