It’s time for the annual ASH meeting, always a showcase for cutting-edge clinical data in competitive areas like blood cancers and hemoglobinopathies.
The weekend slate at ASH did not disappoint, featuring several updates in the CAR-T space. Notably, two programs from less well-known players caught my attention, both employing fresh-in/fresh-out manufacturing (non-cryopreserved starting material and final product). While intriguing, these approaches leave lingering questions about their scalability and commercial potential.
Miltenyi Biomedicines’ Zamto-Cel
First up is Miltenyi’s little-known biomedicines division, with Zamto-Cel, the company’s CD19/20 CAR-T in the pivotal P2 DALY II USA study in 3L+ DLBCL. Miltenyi Biotec is obviously one of the leaders in the cell therapy manufacturing device space. Many cell therapy companies utilize the CliniMACS Prodigy system. Miltenyi Biomedicines is a subsidiary of the device giant focused on advancing largely CAR-T programs, although Zamto-Cel is by far the most advanced.
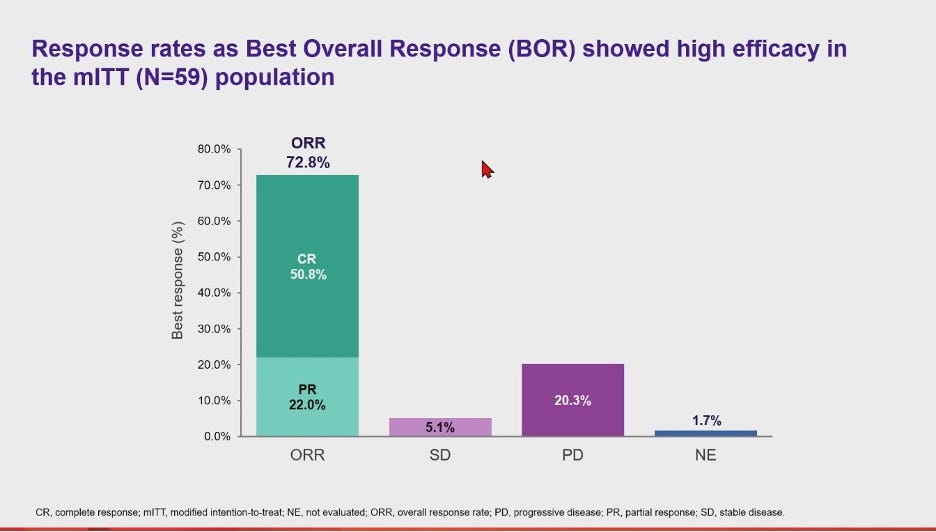
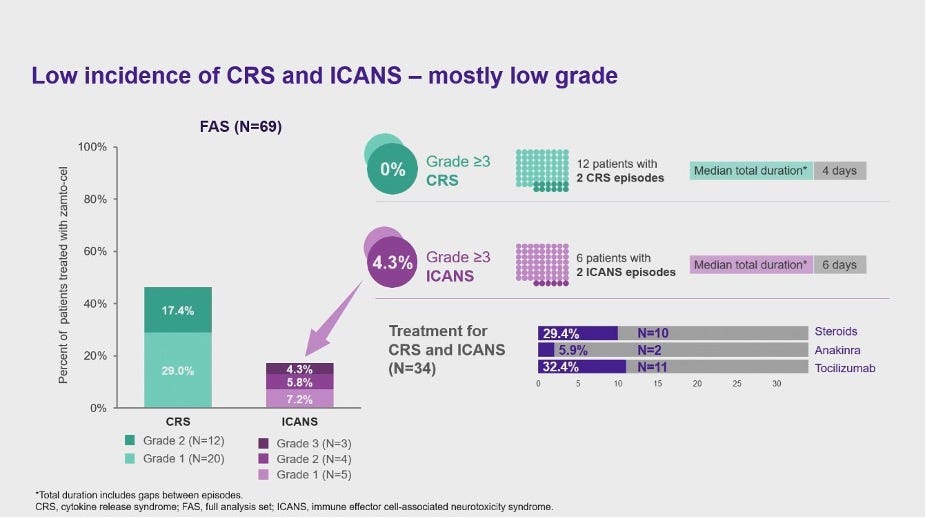
In the interim P2 data readout, Zamto-cel demonstrated an efficacy profile in line with YESCARTA’s, but with lower incidences of CRS and ICANS. Two-targets are maybe showing to better than one, with the median PFS registering at 9 months (vs. ~6 months in YESCARTA’s ZUMA-1), but it is still too early to tell whether the additional targeting is leading to an efficacy advantage.
Zamto-Cel was pioneered by Dr. Nirav Shah from Medical College of Wisconsin, who conducted much of the early work for this Fresh-in/Fresh-out approach in single-center P1 studies. The key differentiator for this approach is the fac that there is no cryopreservation utilized in this process, which Miltenyi and Shah say enables a “true 14-day turnaround time” and minimizes the need for bridging therapy.