ASH 2023 Zeitgeist Review: Winners and Losers
Reviewing some of the buzzy data coming out of ASH and teasing out winners, losers, a data readout that is "somewhere in between", and a couple of under the radar readouts that didn't make the news.
Introduction
The annual ASH meeting took place this past week (December 9th to 12th). Having been someone that has attended pretty much every Oncology conference under the sun, ASH is by far my favorite meeting. The proverbial on base percentage for impactful and interesting data at the conference is always high. The meeting is extremely well run, it’s less of a zoo than ASCO, and being in San Diego every other year is great too.
For this week’s edition of BPS, I am reviewing the zeitgeisty data and headlines coming out of ASH and providing a biotech insider’s assessment (aka my own infallible 🙃assessment) as to who is a winner and a loser coming out of the conference. This is more of a mid/long term strategic view on key data readouts and downstream implications, rather than “wow look at whose stock price went up/down so much after that data release!”. BPS subscribers know that often, the markets over/under react in the short term to catalysts, and the fundamentals of a product’s profile, its competitive environment, and the strategy and execution of the company developing it are what will matter most in the long term.
Winners
Allo and Auto CD19 CAR-T Co’s, Bispecific CD19xCD3 Co’s
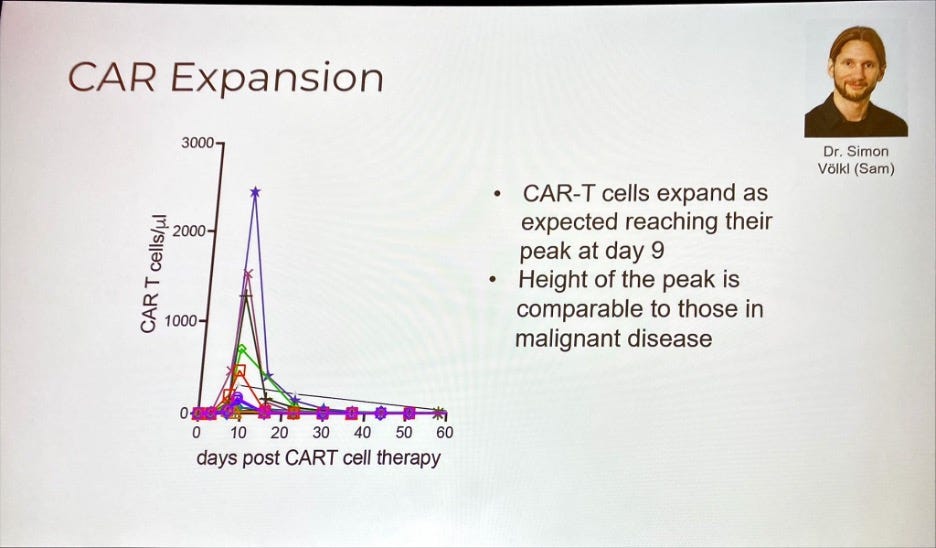
CD19 CAR-T Cells in Refractory Systemic Autoimmune Disease: Single-Center Data from Germany
At least on BioTwitter, these update data seemed to be the main headliner of ASH. We knew from the abstract that 15/15 patients with lupus, myositis, or systemic sclerosis received CD19 CAR-T and remain in remission without need for additional immunosuppressive therapy at a median follow-up of 15 months. That’s impressive on its own. What we didn’t get to see were the photos of just how powerful CD19 CAR-T therapy is in this disease group (see image below):
The patient profiled here looks like two different people entirely in the side-by-side. It’s reminiscent of before/after pictures you would see in a late-night infomercial for some new exercise equipment, only real. This patient has made it out to 24 months without a relapse. Notably, patients on the study did experience infectious complications, which makes sense, given they were without B-cells for some time.
But that wasn’t the part of this data readout that caught my eye. It was the fact that to achieve these remarkable results, the CAR-T cells did not need to persist in the patients’ bodies for very long – less than 20 days according to the data, with patients regaining healthy B-cells after about 120 days (see above).
So, what does this mean for the biotech players developing therapies in this space? Clearly auto CAR-T players like Cabaletta and Kyverna feel as though their lead programs, basically doing the same thing, are more de-risked. However, I think the folks who are really smiling are the allo CD19 CAR-T and bispecific CD19xCD3 antibody companies. If you can get extremely durable remissions in heavily pre-treated refractory patients all with cells that only persist for 20 days (and peak at 10 days), such as this German data showed, perhaps you don’t need an autologous CAR-T at all? A much more cheaply and easily manufactured allo’ CAR-T could potentially persist for long enough needed to generate the same benefit, without the patient needing to wait 2.5-3 weeks to get their cells delivered.
Do not be surprised if these data push the CD19 allo’ players like Caribou and Allogene, to follow CRISPR Therapeutics’ lead and move faster down the autoimmune route. Better yet, a CD19xCD3 bispecific antibody might be able to deplete B-cells to the same degree, driving the same benefit, without the need for lymphodepletion, at far less cost, and (🤫) likely far less payer pushback. For company’ with CD19xCD3 bispecifics (👀 at Amgen, AstraZeneca, Cullinan) who are paying attention, this should be the green light they need to accelerate development of clinical stage bispecifics into autoimmune disease.